

PROCRIT® is indicated to reduce the need for allogeneic RBC transfusions among patients with perioperative hemoglobin (Hb) >10 to ≤13 g/dL who are at high risk for perioperative blood loss from elective, noncardiac, nonvascular surgery. PROCRIT® is not indicated for patients who are willing to donate autologous blood preoperatively.1
PROCRIT® has not been shown to improve quality of life, fatigue, or patient well-being.
PROCRIT® is not indicated for use:
PROCRIT® reduced transfusion rates with both weekly and daily dosing regimens2,3
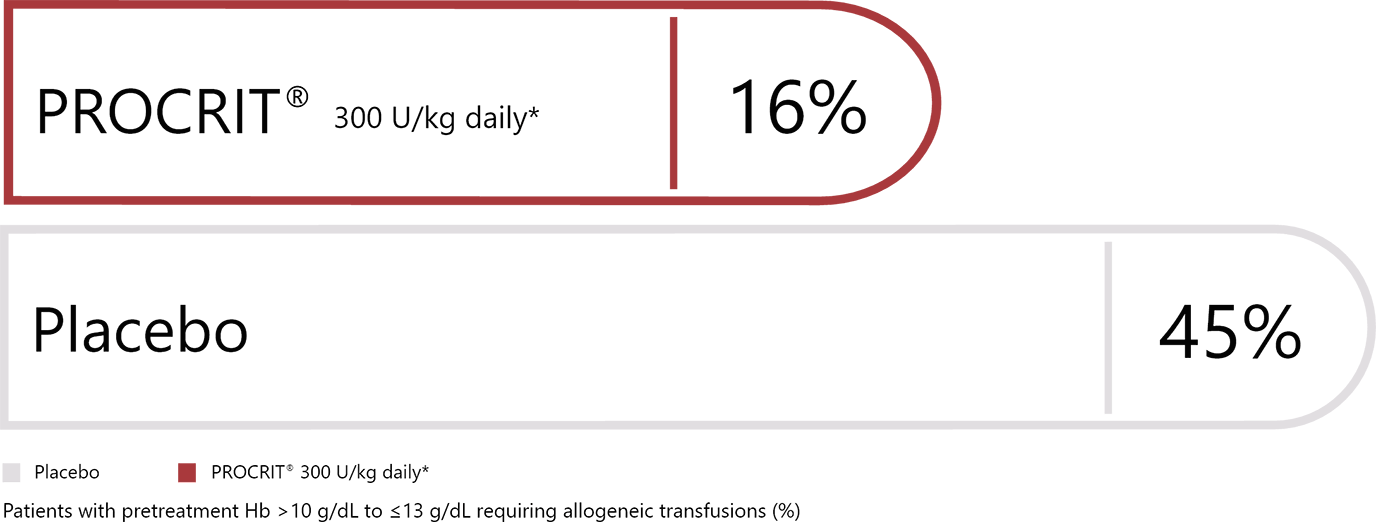
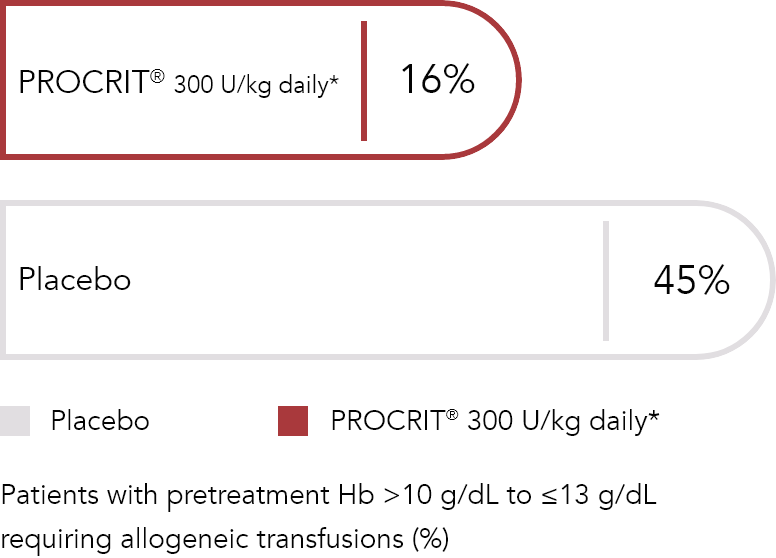
Daily dosing vs placebo1,2
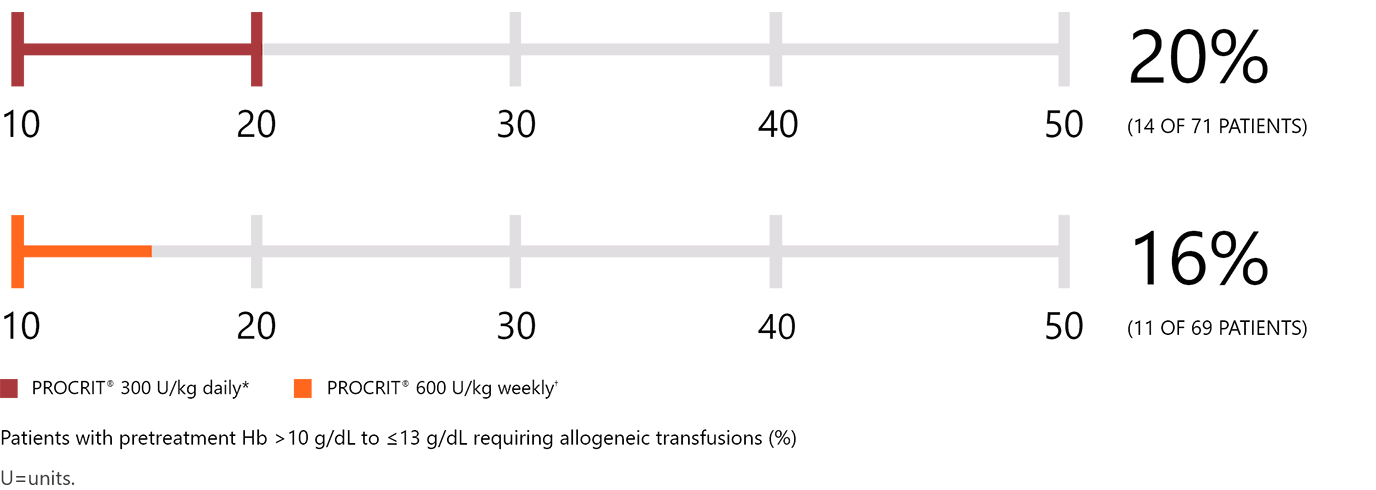
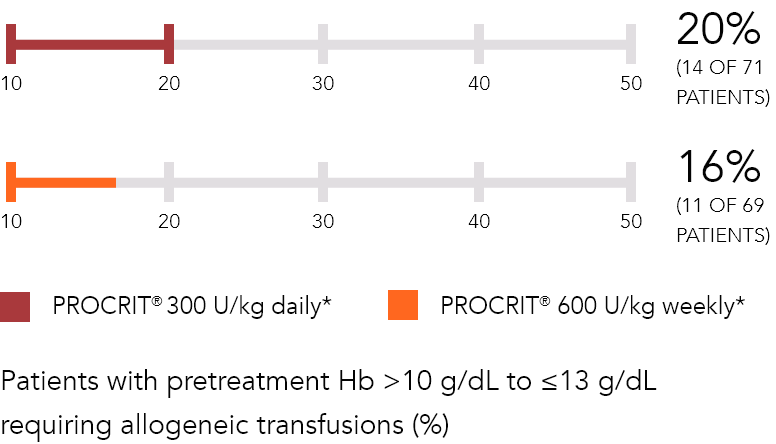
Daily dosing vs weekly dosing1,3
| STUDY | PATIENTS | DESIGN | TREATMENT | ENDPOINTS |
|---|---|---|---|---|
| de Andrade et al2 |
|
|
|
|
| Goldberg et al3 |
|
|
|
|
| STUDY | de Andrade et al2 | Goldberg et al3 |
|---|---|---|
| PATIENTS |
|
|
| DESIGN |
|
|
| TREATMENT |
|
|
| ENDPOINTS |
|
|
*Daily regimen (100 or 300 U/kg SC): for 15 days, starting 10 days prior to, day of, and 4 days after surgery.2,3
†Weekly regimen (600 U/kg SC): once weekly, beginning 3 weeks prior to surgery, with fourth dose on day of surgery.3
SC=subcutaneously.
Mean Hb values in patients with Hb >10 g/dL to ≤13 g/dL
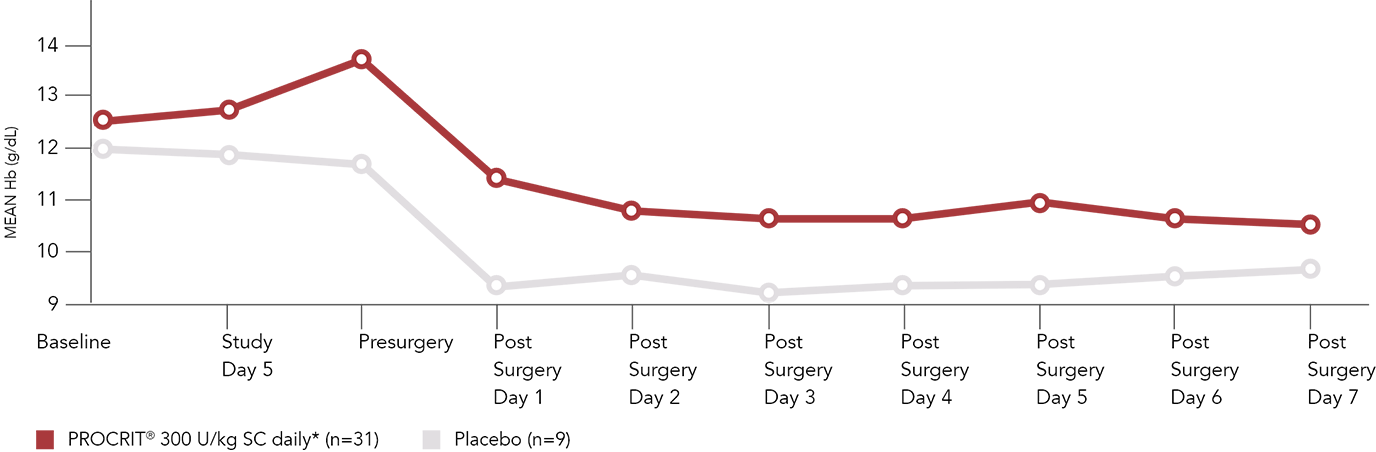
Adapted with permission from de Andrade et al. Am J Orthop. 1996;25:533-542.
Mean reticulocyte counts in patients with Hb >10 g/dL to ≤13 g/dL
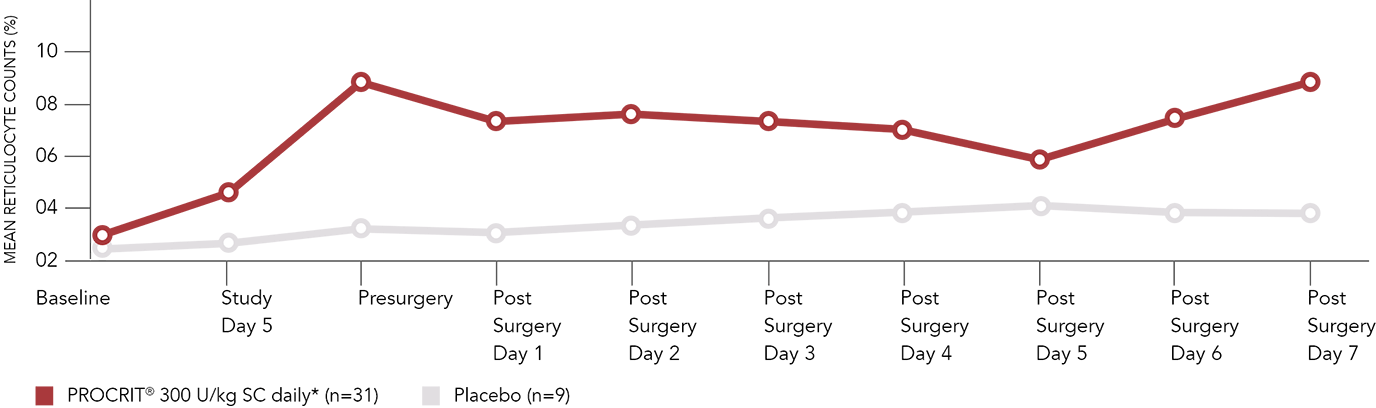
Adapted with permission from de Andrade et al. Am J Orthop. 1996;25:533-542.
WARNINGS: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
Perisurgery:
Due to increased risk of deep venous thrombosis (DVT), DVT prophylaxis is recommended.
Expand the Important Safety Information at the bottom of the page to see the complete Boxed Warnings.
The adverse reactions with a reported incidence of ≥1% in PROCRIT®-treated patients that occurred at a higher frequency than in placebo-treated patients are shown in the table below:
Adverse reactions in surgery patients1
| EVENT | |||||
|---|---|---|---|---|---|
| NAUSEA | 47% | 43% | 45% | 45% | 56% |
| Vomiting | 21% | 12% | 14% | 19% | 28% |
| Pruritus | 16% | 16% | 14% | 12% | 21% |
| Headache | 13% | 11% | 9% | 10% | 18% |
| Injection site pain | 13% | 9% | 8% | 12% | 11% |
| Chills | 7% | 4% | 1% | 1% | 0% |
| Deep vein thrombosis|| | 6% | 3% | 3% | 0% | 0% |
| Cough | 5% | 4% | 0% | 4% | 4% |
| Hypertension | 5% | 3% | 5% | 5% | 6% |
| Rash | 2% | 2% | 1% | 3% | 3% |
| EDEMA | 1% | 2% | 2% | 1% | 3% |

‡Study including patients undergoing orthopedic surgery treated with PROCRIT® or placebo for 15 days.
§Study including patients undergoing orthopedic surgery treated with PROCRIT® 600 U/kg SC weekly x 4 weeks or 300 U/kg SC daily x 15 days.
||Deep vein thromboses were determined by clinical symptoms.