

PROCRIT® is indicated for the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy.
PROCRIT® has not been shown to improve quality of life, fatigue, or patient well-being.
PROCRIT® is not indicated for use:
Following initiation of therapy and after each dose adjustment, monitor hemoglobin (Hb) weekly until the Hb level is stable and sufficient to minimize the need for RBC transfusion.
In pregnant women, lactating women, neonates, and infants use only single-dose vials (the benzyl alcohol–free formulation).
Initiate PROCRIT® in patients on cancer chemotherapy only if Hb is <10 g/dL, and if there is a minimum of 2 additional months of planned chemotherapy.
Use the lowest dose of PROCRIT® necessary to avoid RBC transfusions.
IV=intravenous; QW=once weekly; SC=subcutaneous; TIW=3 times weekly; U=units.
| Dose Reduction | |
|---|---|
| Hb increases >1 g/dL in any 2-week period | Reduce dose by 25% |
| Hb reaches a level needed to avoid RBC transfusion | Reduce dose by 25% |
| Hb exceeds a level needed to avoid RBC transfusion | Withhold dose. Reinitiate at a dose 25% below the previous dose when Hb approaches a level where RBC transfusions may be required |



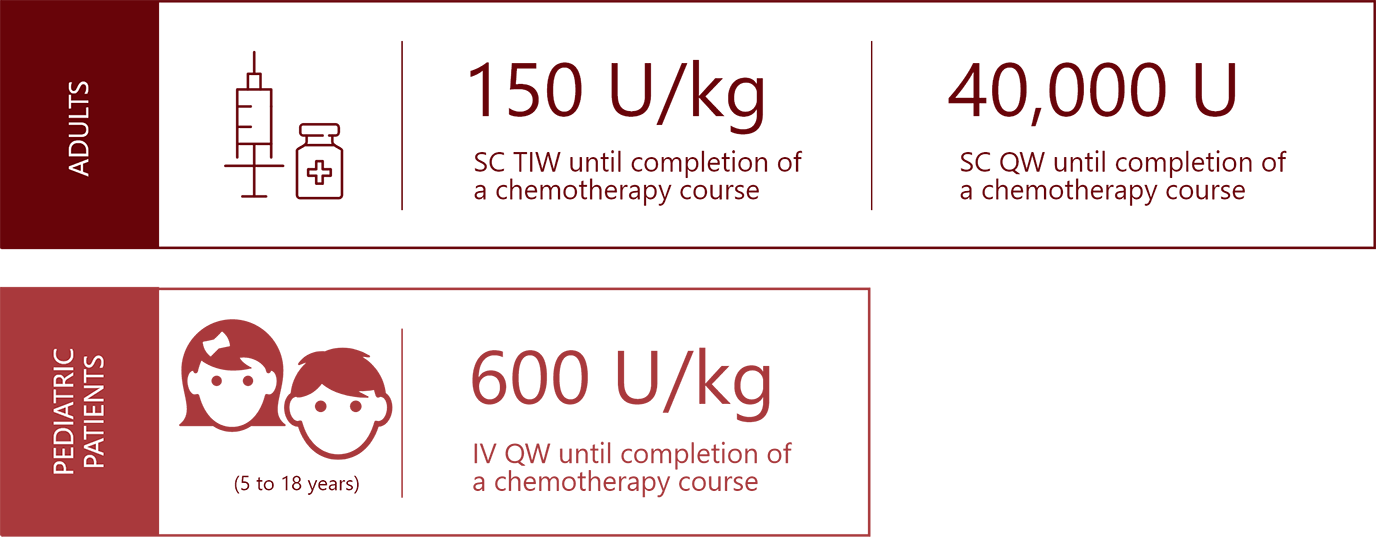
40,000 U
subcutaneous
QW
until completion of a chemotherapy course
150 U/kg
subcutaneous
TIW
until completion of a chemotherapy course


Reduce dose by 25% if:
Withhold dose if:


If QW dosing:
Assess response after Week 4


If TIW dosing:
Assess response after Week 4


If Hb increases by <1 g/dL and remains below 10 g/dL:
If Hb rise is ≥1 g/dL:
If Hb increases by <1 g/dL and remains below 10 g/dL:



Manage therapy
PROCRIT® is contraindicated in patients with uncontrolled hypertension, pure red cell aplasia (PRCA) that begins after treatment with PROCRIT® or other erythropoietin protein drugs, or serious allergic reactions to PROCRIT®. PROCRIT® from multiple-dose vials contains benzyl alcohol and is contraindicated in neonates, infants, pregnant women, and lactating women.1 Please see the FULL PRESCRIBING INFORMATION to learn more.